Serum Institute of India gets DGCI not to Resume the Clinical Trials
September 16, 2020 11:12
(Image source from: Navbharattimes.indiatimes.com)
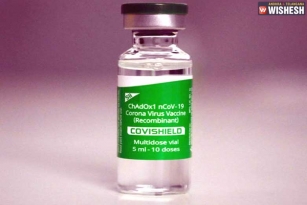
Serum Institute of India gets DGCI not to Resume the Clinical Trials:- The Drugs Controller General of India (DGCI) headed by Dr V G Somani granted permission for the Serum Institute of India to resume the human clinical trials of the coronavirus vaccine that is developed by the University of Oxford in association with the Serum Institute of India. The trials are suspended after one of the volunteers faced minor health issues. The DGCI asked the Serum Institute of India to take extra care during the trials when screening and providing information. Serum Institute of India will have to submit the details of the medication to the DGCI that is used with the protocol. On September 11th, the DGCI asked Serum Institute of India to suspend the trials.
AstraZeneca which is working with Oxford and SII has been conducting the trials for the vaccine in various countries of the globe. All these trials resumed in other countries as well recently. Medicines Health Regulatory Authority's (MHRA) confirmed that the trials are safe after which AstraZeneca and the University of Oxford resumed the clinical trials for the coronavirus vaccine in the United Kingdom. SII submitted the recommendations of Data and Safety Monitoring Board (DSMB), UK and DSMB. The participant information sheet was submitted by SII to the DGCI. The safety followup for seven days post first vaccination too was submitted by the SII.