(Image source from: Business-standard.com)
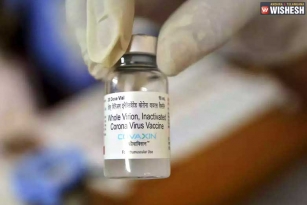
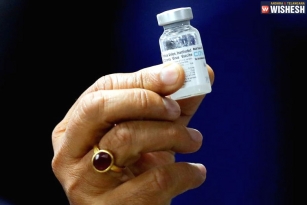
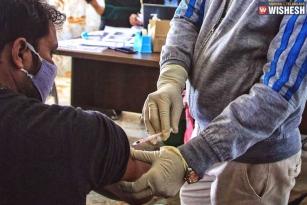
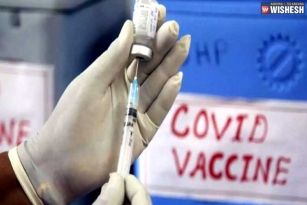
Bharat Biotech Starts Human Trials For Coronavirus Vaccine:- Bharat Biotech already announced that the human clinical trials for the vaccination of COVID-19 would start in July. The first phase of human trials started today at Rohtak's Post-Graduate Institute of Medical Sciences. Haryana Health Minister Anil Vij informed the news through his twitter handle. "Human trial with Corona vaccine (COVAXIN) of Bharat Biotech started at PGI Rohtak today. Three subjects were enrolled today. All have tolerated the vaccine very well. There were no adverse efforts" posted Anil Vij today.
Human trial with Corona vaccine (COVAXIN) of Bharat Biotech started at PGI Rohtak today. Three subjects were enrolled today. All have tolerated the vaccine very well. There were no adverse efforts.
— ANIL VIJ MINISTER HARYANA (@anilvijminister) July 17, 2020
It is heard that three subjects were enrolled and the results have been positive. Bharat Biotech is the first pharmaceutical company in the country to get approval to start the clinical trials for the vaccine named Covaxin. As per the reports, there are seven anti-coronavirus vaccines developed in the country that are in various states and some of them received approval for clinical trials. Zydus said that they got the approval for the human trials earlier this month. The ICMR is closely monitoring the process. The clinical trials will start in Hyderabad's NIMS soon. The volunteers are finalized and NIMS is expected to receive the drug very soon.